Background
Non-secretory multiple myeloma (NSMM), 1-2% of all multiple myeloma (MM) cases, was traditionally defined by the absence of detectable M-Spike in serum or urine. Advancements in diagnostic methodologies have revealed that a proportion of NSMMs are, in fact, more accurately categorized as oligo-secretory, highlighting the dynamic nature of MM and its classification. Disease evolution, especially towards less secretory types, significantly impacts monitoring, progression, and prognosis. This study examines MM secretion patterns (SP) and evolution, particularly NSMM and oligo-secretory types, comparing current classification with medical notes.
Methodology
Using the HealthTree Cure Hub for Multiple Myeloma (PMID: 35271305), we analyzed real-world data from 140 patients. SP evolution was assessed as Secretory (SC), Light Chain Only Oligosecretory (LCO), Heavy Chain Oligosecretory (HCO), and True Non-secretory (TNSC) at diagnosis and last lab follow-up. TNSC patients showed no myeloma activity on the M-protein, and light chain assay. LCO patients exhibited activity only on their light chain assay. HCO patients met the IMWG criteria for Non-Measurable Myeloma. SC patients didn't meet the criteria for the other categories. Changes to less secretory patterns were recorded based on office notes, indicating a relapse diagnosis. The chi-square test of independence assessed the association between secretion types at diagnosis and current state and the concordance between IMWG criteria and medical notes. Kaplan-Meier ‘Time to Next Treatment’ (TTNT) analysis was implemented for the first line of therapy to assess the impact of secretion type at diagnosis and currently. Patients whose SP could not be determined were excluded.
Results
The mean age was 65±8.5 y.o., and 56.5% were female. Of the 122 patients that had an mSMART stage available, 13% were high-risk, and 6% were double-hit.
At their last follow-up, the SPs of the 140 patients analyzed were as follows: 48% SC, 28.5% LCO, 10% HCO, and 13.5% TNSC patients. When comparing SPs at the time of MM diagnosis, 16 patients (11.5%) were found to have changed to a less secretory type. This change took a mean of 66.5 months from the time of diagnosis, with SC-to-HCO (43.25 mo) and HCO-to-TNSC (34.5 mo) being the fastest and SC-to-TNSC being the slowest (96.5 mo). Among the 73 patients with LCO, HCO, or TNSC patterns, 75.3% had their SP accurately reported, 12.3% had it incorrectly classified, and 12.3% were not classified.
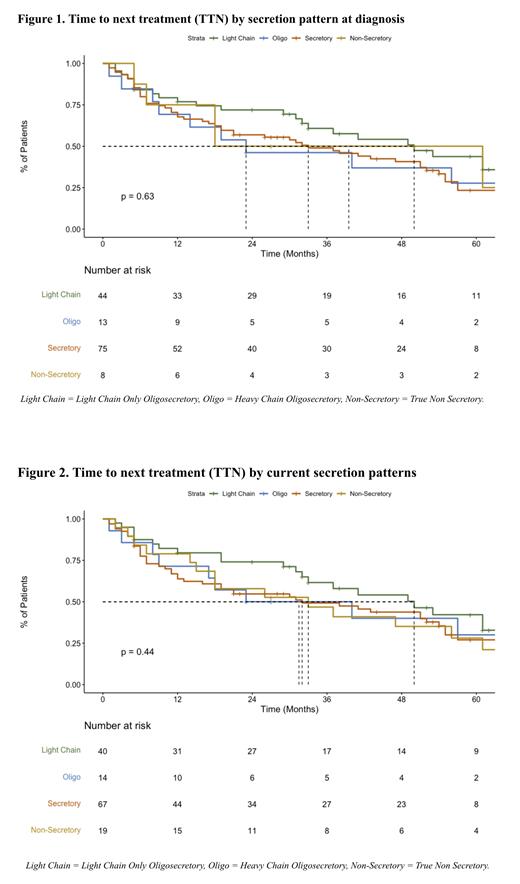
The median TTNTs of the observed events at the time of the last follow-up using the SP at diagnosis were: SC (39 mo), LCO (50 mo), HCO (23 mo), and TNSC (33 mo) ( p = 0.63). The median TTNTs, when using the current SP, were: SC (32 mo), LCO (50 mo), HCO (31.5 mo), and TNSC (33 mo) ( p = 0.44). After two years of follow-up, the TTNT survival rates using the SP at diagnosis were: SC (53.3%), LCO (65.9%), HCO (38.5%), and TNSC (50%). The TTNT survival rates, when using the current SP, were: SC (50.7%), LCO (67.5%), HCO (42.9%), and TNSC (57.9%).
Conclusions
Our study elucidates the dynamic evolution of MM SPs, with 11.5% of patients transitioning to less secretory types. Understanding these patterns is pivotal for effective disease monitoring and therapy planning.
While the limited number of NSMM patients prevents definitive conclusions, our findings align with existing literature suggesting that the NSMM at onset does not confer additional risk (PMID:28894562). Interestingly, we found no retrospective impact of SP evolution at disease onset, which has been linked to a worse prognosis at relapse.
However, one of four patients had inaccurate or missing SP information in their office notes, highlighting the need for improved documentation and awareness. Future research should explore the clinical implications of these findings and continue to refine MM classifications according to the testing technology available.
Disclosures
Ahlstrom:Janssen: Other: Patient Advocacy Committee; Takeda Oncology: Other: Patient Advocacy Committee; Pfizer: Other: Patient Advocacy Committee; BMS: Other: Patient Advocacy Committee; Sanofi: Other: Patient Advocacy Committee. Hydren:Adaptive Biotechnologies: Research Funding; Pfizer: Research Funding; GSK: Research Funding; Regeneron: Research Funding; Janssen Oncology: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal